- 主页
- 激酶检测技术支持门户
- 激酶检测支持资料
- Capillary Electrophoresis 激酶检测支持
Capillary Electrophoresis 激酶检测支持
迁移率变化分析(Mobility Shift Assay,简称 MSA)作为一种在酶促反应中分离底物与产物的方法,已广泛应用于药物研发和科研领域超过 20 年。在 Carna,我们对底物与产物分离及分析所涉及的各项要素进行了优化,例如缓冲液条件和分离参数,从而实现使用 SCIEX 的 PA800 系统进行 MSA 检测。这些优化后的毛细管电泳检测条件也被应用于我们自研蛋白的质量控制测试中。
迁移率变化分析(Mobility Shift Assay,简称 MSA)是一种通过底物与产物在酶促反应中迁移率差异进行分离和检测的分析方法。当激酶等酶作用于底物时,磷酸化会改变产物的电荷,从而使底物与产物之间产生电荷差异。这种差异会导致在电泳过程中迁移速度的变化,从而可用于检测激酶的磷酸化活性。
Carna 使用 SCIEX 的 PA800 系统作为检测平台,提供针对迁移率变化分析(MSA)/毛细管电泳激酶分析的优化检测条件。
如需查询下表所列各 FL 标记肽底物对应的激酶信息,请参见FL 标记肽底物清单页面;如有进一步需求,欢迎通过此处或发送邮件至 info@sb.carnabio.com 与我们联系。
| Experimental/lab equipment | Manufacturer | Product number |
|---|---|---|
| PA 800 Plus | SCIEX | A66528 |
| Bare Fused-Silica Capillary | SCIEX | 338451 |
| Polypropylene 96 well microplate | Greiner | 655201 |
| Substrate Name (FL-labeled Peptide Substrate) |
Substrate Cat. No. |
Voltage (KV) | Duration (min) | Pressure (psi) | Pressure direction | Peak Charge Figure |
|---|---|---|---|---|---|---|
| ABLtide | MSSU16 | 25 | 2 | 0.5 | Forward | 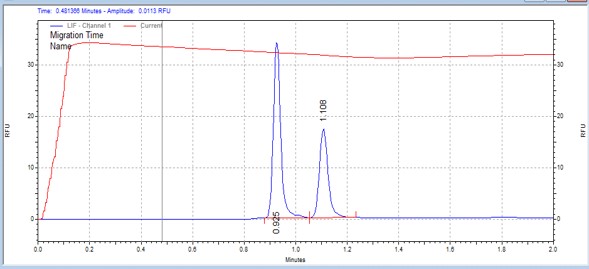 |
| AMARA peptide | MSSU28 | 25 | 2 | 0.5 | Forward | 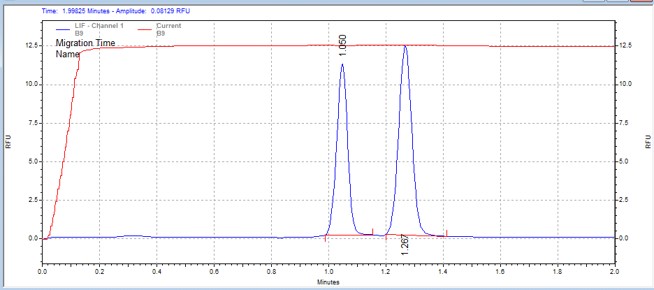 |
| Blk/Lyntide | MSSU04 | 25 | 2 | 0.7 | Forward | 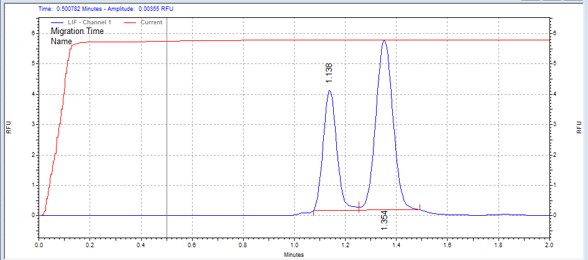 |
| CDC25ctide | MSSU21 | 25 | 2 | 0.8 | Forward | 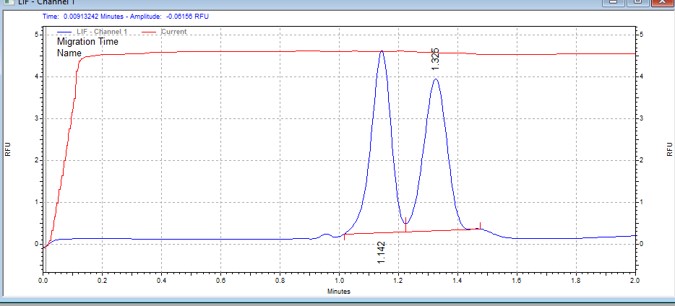 |
| CDK7 peptide | MSSU11 | 25 | 2 | 0.5 | Forward | 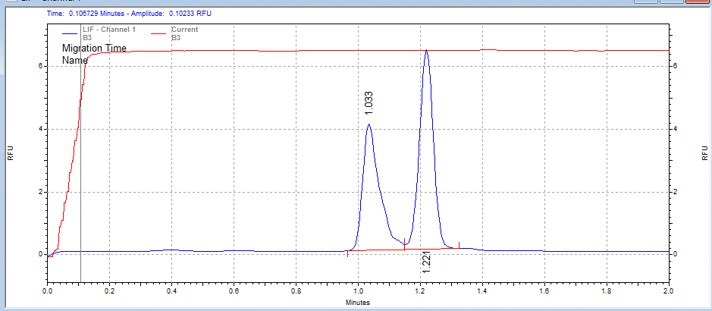 |
| CDK9 substrate | MSSU29 | 25 | 2 | 0.7 | Forward | 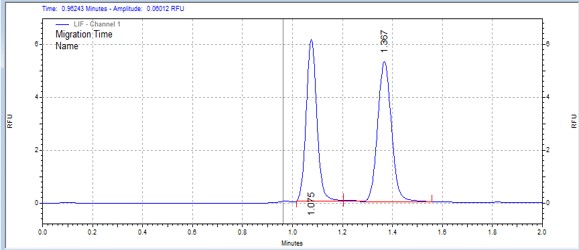 |
| CHKtide | MSSU08 | 25 | 3 | 0.5 | Reverse | 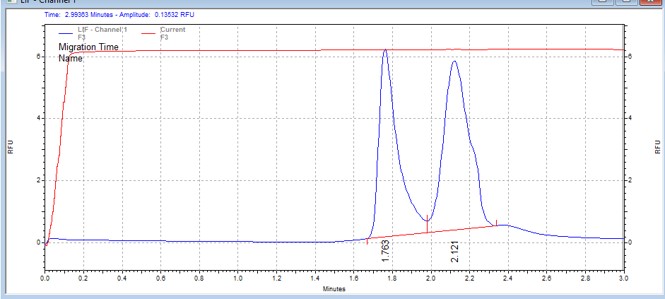 |
| CK2tide | MSSU25 | 25 | 2 | 0.8 | Forward | 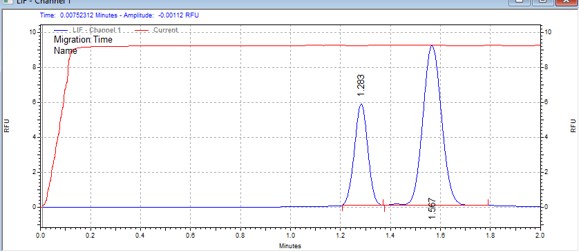 |
| CKtide | MSSU12 | 25 | 2 | 0.5 | Forward | 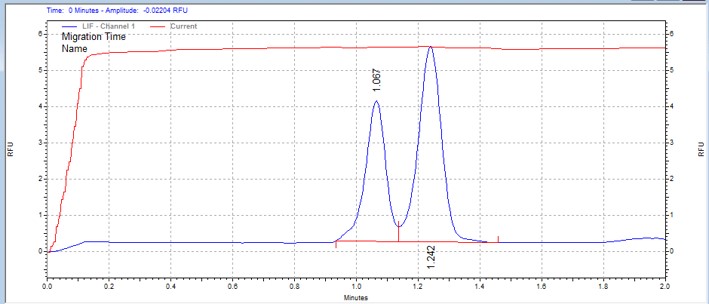 |
| CREBtide-p | MSSU22 | 25 | 2 | 0.5 | Forward | 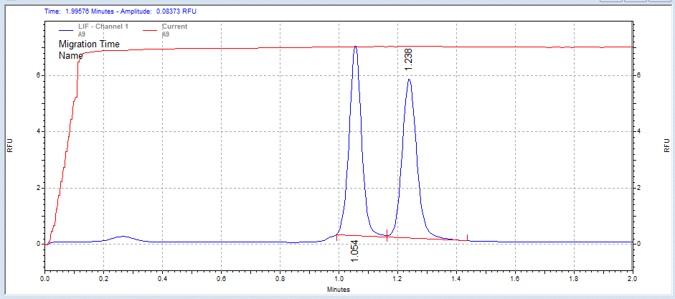 |
| Crosstide | MSSU19 | 25 | 2 | 0.5 | Forward | 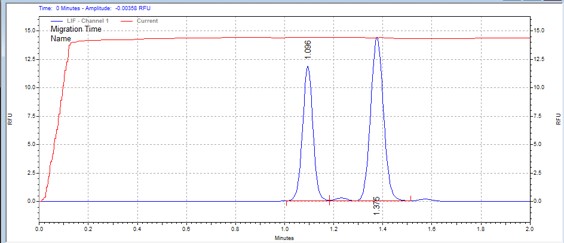 |
| CSKtide | MSSU02 | 25 | 2 | 0.5 | Forward | 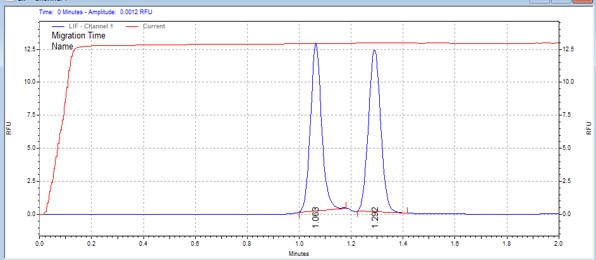 |
| CTD3 peptide | MSSU30 | 25 | 2 | 0.5 | Forward | 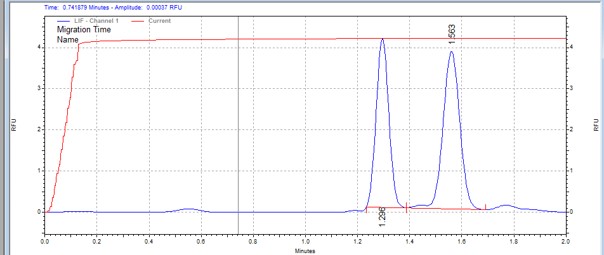 |
| DAPK1tide | MSSU17 | 16 | 3 | 0 | Forward | 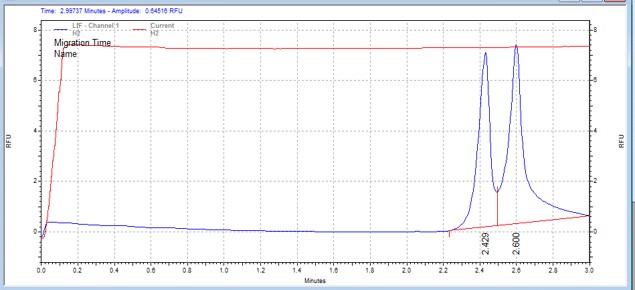 |
| DYRKtide-F | MSSU06 | 25 | 2 | 0.2 | Forward | 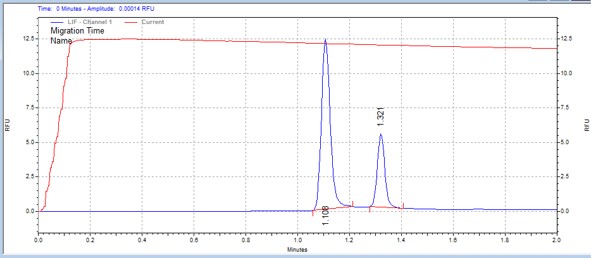 |
| EEF2Ktide | MSSU40 | 25 | 2 | 0.5 | Forward | 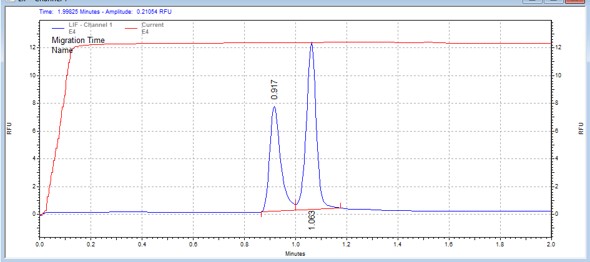 |
| EGFR-derived peptide | MSSU31 | 25 | 2 | 0.8 | Forward | 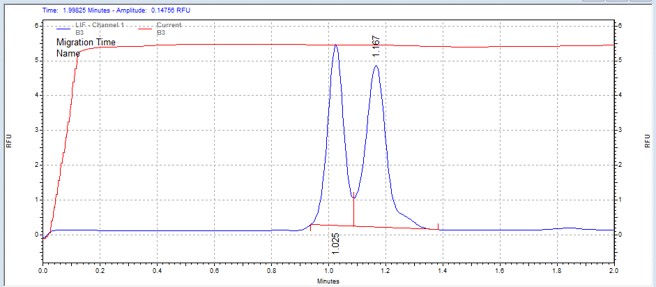 |
| GS peptide | MSSU05 | 25 | 1.5 | 0.8 | Forward | 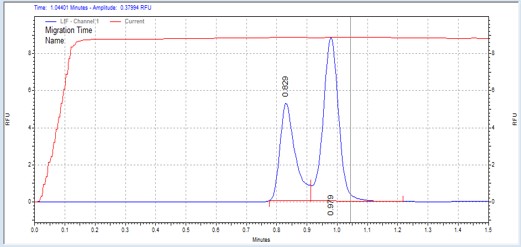 |
| H2A peptide | MSSU47 | 25 | 2 | 0.5 | Forward | 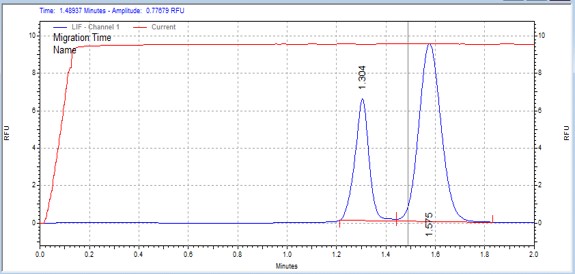 |
| Histone H3 peptide | MSSU23 | 25 | 2 | 0.3 | Forward | 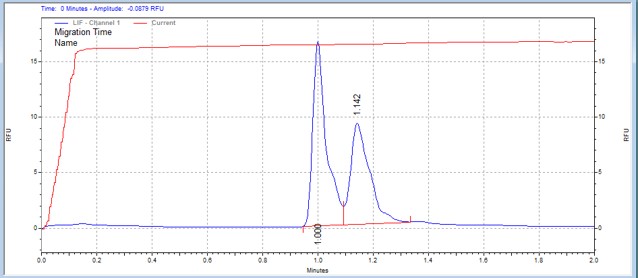 |
| IRAK1 peptide* | MSSU41 | 25 | 1.4 | 0.5 | Forward | 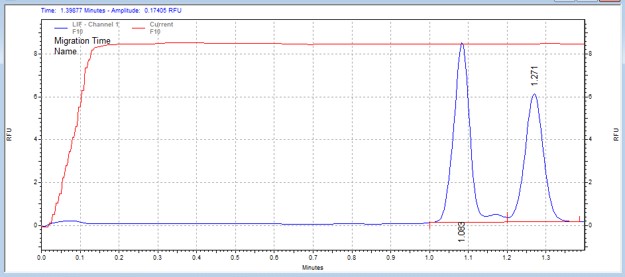 |
| IRS1 | MSSU07 | 25 | 2 | 0.5 | Forward | 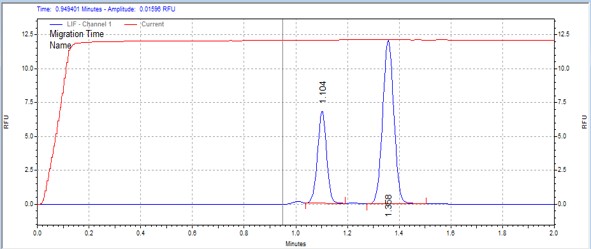 |
| IκBα peptide | MSSU42 | 25 | 2 | 0.8 | Forward | 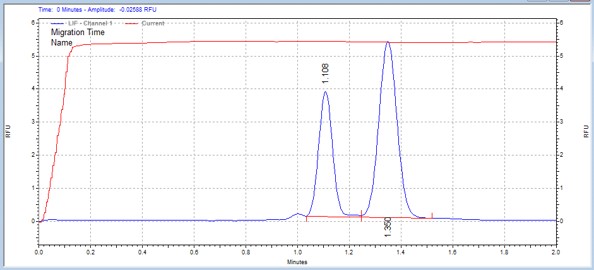 |
| JAK1 substrate peptide | MSSU32 | 25 | 2 | 0.8 | Forward | 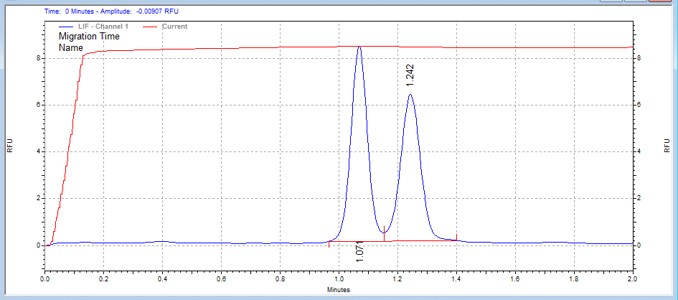 |
| Kemptide | MSSU09 | 25 | 2 | 0.8 | Forward | 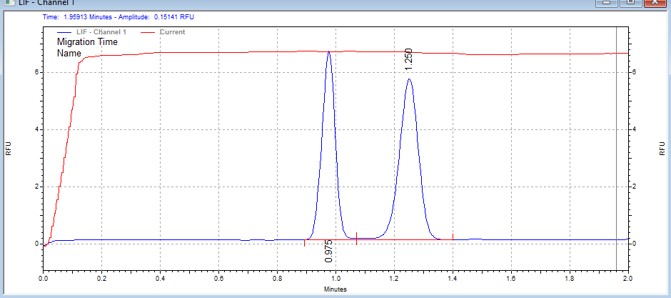 |
| LIMKtide | MSSU24 | 25 | 2 | 0.5 | Forward | 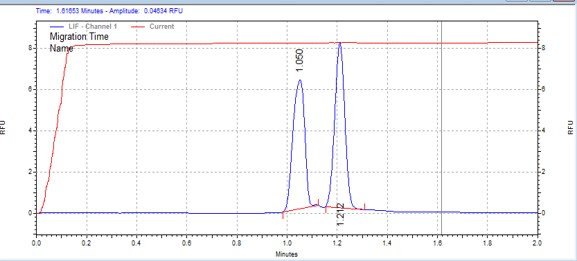 |
| LRRKtide | MSSU48 | 25 | 2 | 0 | Reverse | 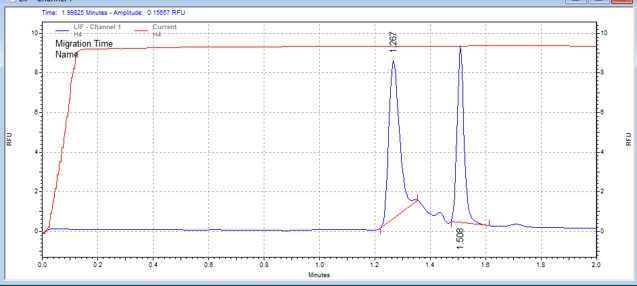 |
| MCM2 peptide | MSSU33 | 25 | 2 | 0.5 | Forward | 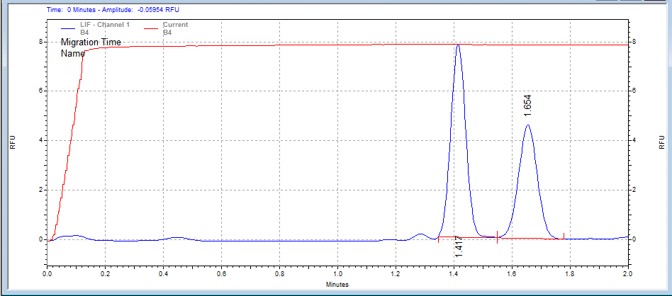 |
| MLCtide | MSSU43 | 25 | 2 | 0.5 | Forward | 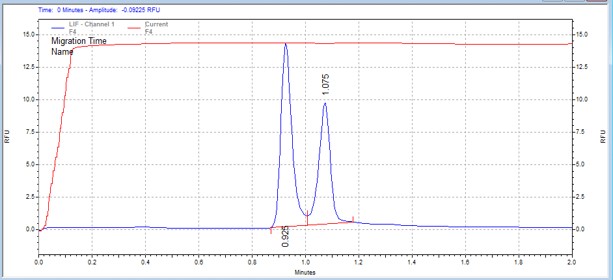 |
| Modified Erktide | MSSU03 | 25 | 2 | 0.5 | Forward | 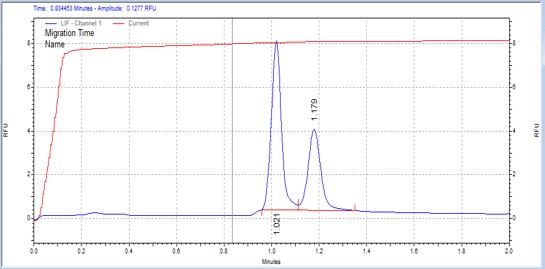 |
| Modified Histone H1 | MSSU13 | 25 | 2 | 0.5 | Forward | 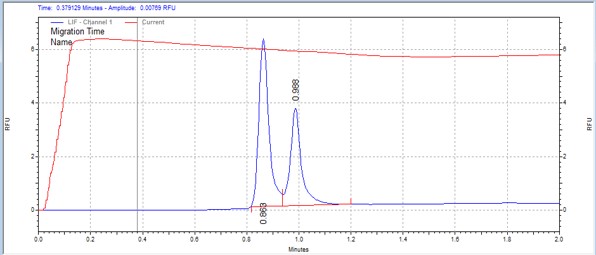 |
| Modified IκBα-derived peptide | MSSU34 | 25 | 2 | 0.8 | Forward | 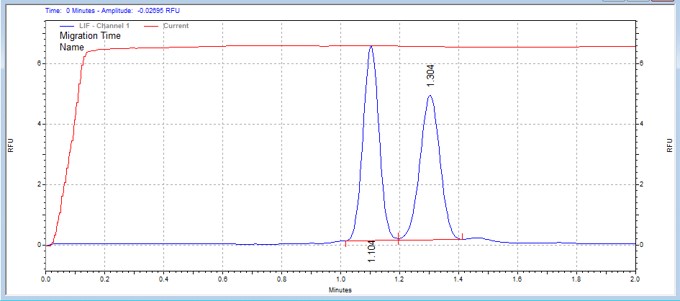 |
| Moesin-derived peptide | MSSU18 | 25 | 2 | 0.8 | Forward | 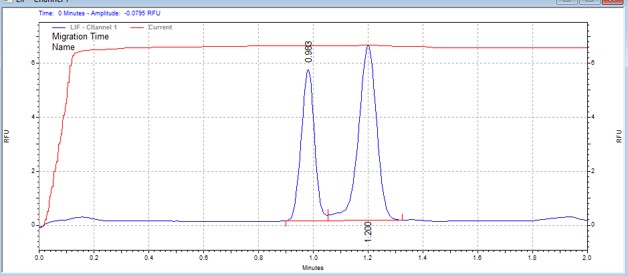 |
| PDHKtide | MSSU35 | 25 | 2 | 0.8 | Forward | 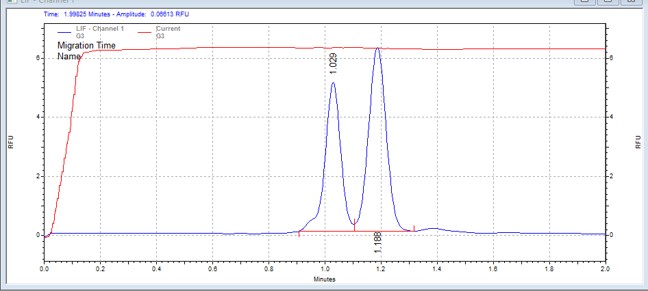 |
| PKC peptide | MSSU10 | 25 | 2 | 0.2 | Forward | 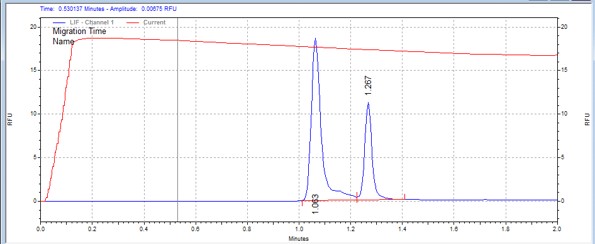 |
| RS peptide | MSSU36 | 25 | 2 | 0.5 | Forward | 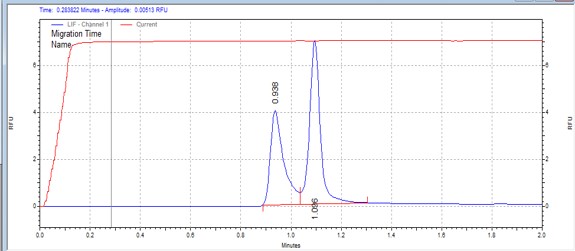 |
| S6K peptide(N-FL) | MSSU20 | 25 | 2 | 0.5 | Forward | 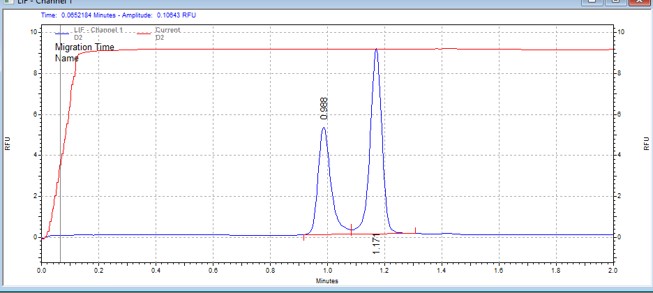 |
| S6K2 peptide | MSSU14 | 25 | 2 | 0.5 | Forward | 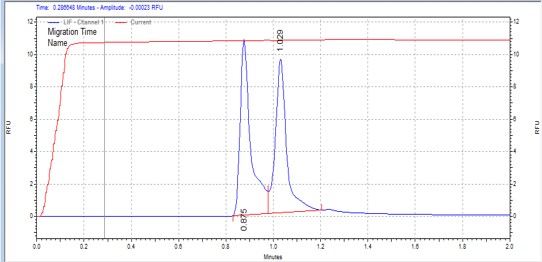 |
| SAMS peptide | MSSU26 | 25 | 2 | 0.5 | Forward | 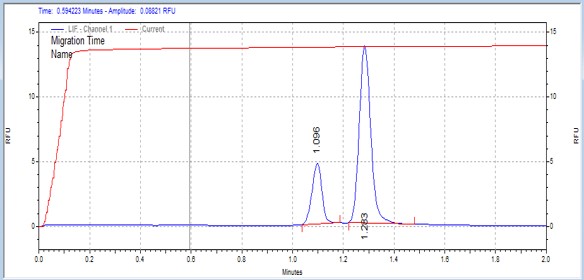 |
| SGKtide | MSSU15 | 25 | 2 | 0.5 | Forward | 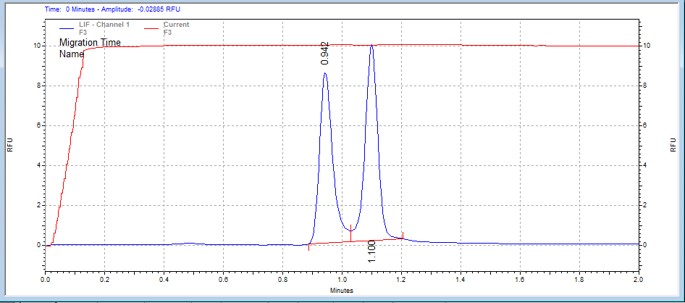 |
| SPAKtide | MSSU27 | 25 | 2 | 0.5 | Forward | 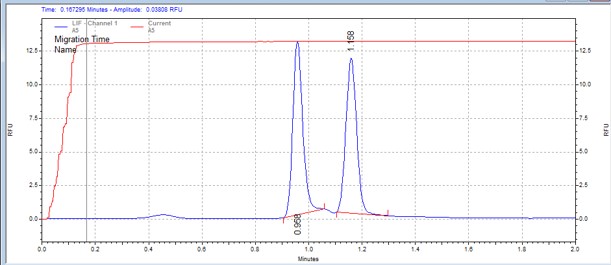 |
| Srctide | MSSU01 | 25 | 2 | 0.5 | Forward | 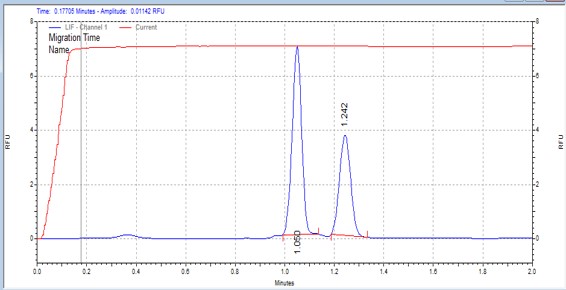 |
| Synapsin peptide | MSSU45 | 25 | 2 | 0.8 | Forward | 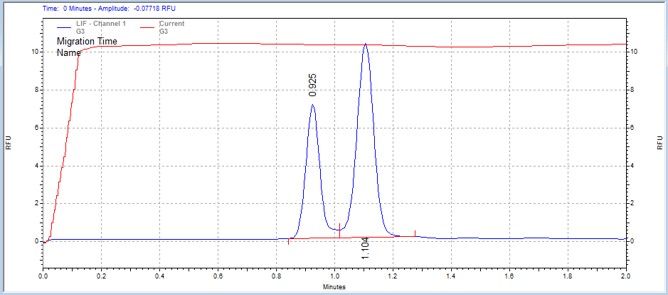 |
| T308tide | MSSU38 | 25 | 2 | 0.5 | Forward | 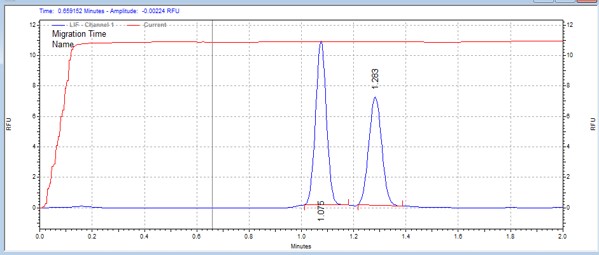 |
| TAOKtide | MSSU46 | 25 | 2 | 0.8 | Forward | 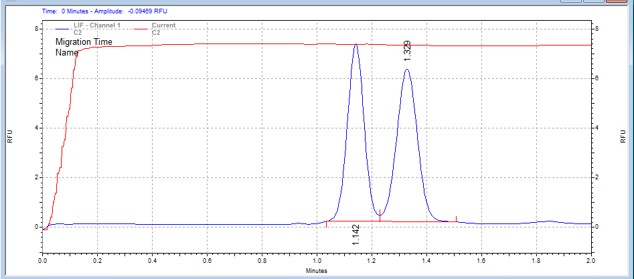 |
| WASP peptide | MSSU39 | 25 | 2 | 0.8 | Forward | 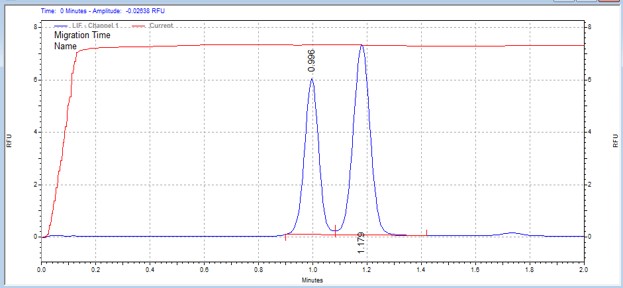 |
分离缓冲液 : 100 mM HEPES pH7.5, 10 mM MgCl2, 0.01 % Triton X-100, 10 mM EDTA-2Na, 1 % DMSO
*消除单孔电泳在约 1.5 min 处出现的杂峰,需追加一步以 Separation Buffer 进行的分离电泳。
