INVESTIGATIONAL BTK INHIBITOR
AS-1763
Wild-type & pan-mutant BTK inhibitor
- TARGET
- BTK (Bruton's tyrosine kinase)
- INDICATION
- Hematological Malignancies
- MODALITY
- Small molecule
- DEVELOPMENT STAGE
- Phase 1b in the U.S.
- CLINICAL TRAIL INFORMATION
- https://clinicaltrials.gov/study/NCT05602363
Mechanism of Action
BTK plays a crucial role in B-cell antigen receptor (BCR) signaling which is essential for B cell development, and BTK has been recognized as a validated therapeutic target for B-cell malignancies including chronic lymphocytic leukemia (CLL). Covalent BTK inhibitors have been appreciated as a promising targeted therapy for patients with B-cell malignancies. However, the emergence of clinical resistance to these covalent BTK inhibitors is becoming serious concerns. BTK C481S mutation predominantly confers this drug resistance by preventing covalent binding of the existing BTK inhibitors. In addition, non-C481 BTK mutations have been reported in patients with CLL during non-covalent BTK inhibitor treatment. These observations underscore the urgent need for a pan-mutant BTK inhibitor that is effective against C481 mutation as well as non-C481 mutations of BTK.
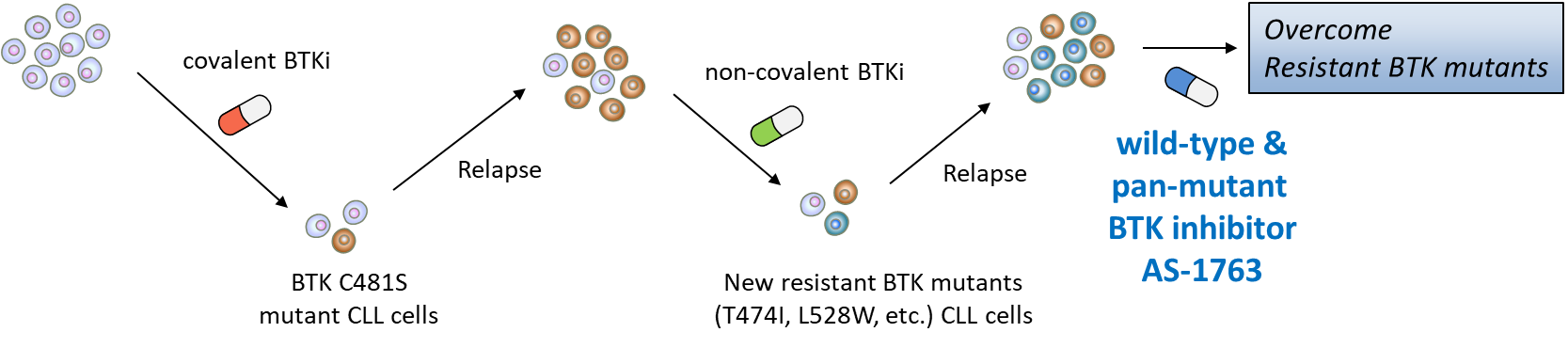
Preclinical Study
AS-1763 is active against emerging resistant mutations (C481S, T474x, T316x, L528x) in BTK to both covalent and non-covalent inhibitors. AS-1763 demonstrated significant antitumor potency in diffuse large B-cell lymphoma (DLBCL) mouse model and showed strong synergy with Bcl-2 inhibitor.
Clinical Study
The Phase 1 single ascending dose study in healthy volunteers has been completed. AS-1763 was well-tolerated and demonstrated a favorable safety profile after single oral doses up to 600 mg.
AS-1763 is currently being evaluated in open-label, multi-center Phase 1b study in patients with CLL/small lymphocytic lymphoma (SLL) and B-cell non-Hodgkin lymphoma (B-cell NHL) who have failed or intolerant to at least two lines of systemic therapy. The study consists of dose escalation part and dose expansion part. The primary objective of the dose escalation part is to determine the maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of AS-1763. Safety profile, tolerability, pharmacokinetics (PK), and preliminary efficacy will be also evaluated as secondary objectives. The dose expansion part will further recruit patients with previously treated CLL/SLL or B-cell NHL to evaluate safety, efficacy, and PK of AS-1763 at multiple doses selected in the dose escalation part and to determine the recommended phase 2 dose (RP2D).
References
- Trial in Progress: A Phase 1b Study of AS-1763, an Oral, Potent and Selective Noncovalent BTK Inhibitor, in Patients with Previously Treated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma or Non-Hodgkin Lymphoma, 65th American Society of Hematology Annual Meeting 2023
- Characterization and Preclinical Evaluation of AS-1763, an Oral, Potent and Selective Noncovalent BTK Inhibitor, in Chronic Lymphocytic Leukemia, 65th American Society of Hematology Annual Meeting 2023
- Safety, pharmacokinetics, and pharmacodynamics of AS-1763, a highly selective, orally bioavailable, non-covalent BTK inhibitor, in healthy volunteers, American Association for Cancer Research Annual Meeting 2022
- Discovery of AS-1763: A Potent, Selective, Noncovalent, and Orally Available Inhibitor of Bruton's Tyrosine Kinase. J Med Chem. 2021 Oct 14;64(19):14129-14141.
