INVESTIGATIONAL BTK INHIBITOR
sofnobrutinib (AS-0871)
Extremely selective non-covalent BTK Inhibitor
- TARGET
- BTK (Bruton's tyrosine kinase)
- INDICATION
- Inflammatory/Immune disorders
- MODALITY
- Small molecule
- DEVELOPMENT STAGE
- Phase 1 in the Netherlands (completed)
Mechanism of Action
BTK is a Tec family tyrosine kinase expressed in B cells and myeloid cell populations including monocytes, macrophages, neutrophils, basophils and mast cells. BTK has a crucial role in B-cell antigen receptor (BCR) signaling during B cell development and activation. In allergic diseases, BTK is a critical enzyme for Fcɛ receptor (FcɛR) signaling in mast cells and basophils to regulate release of chemical mediators such as histamine and leukotrienes. In autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus, aberrant BCR signaling is considered to promote diseases through production of autoantibodies. In addition to BCR signaling, BTK mediates downstream signaling of the Fcγ receptors in myeloid cells to produce inflammatory cytokines such as IL-6 and TNF-α, which would aggravate rheumatoid arthritis (RA) symptoms. Therefore, BTK is being paid attention as an attractive therapeutic target for the treatment of allergic diseases and autoimmune diseases.
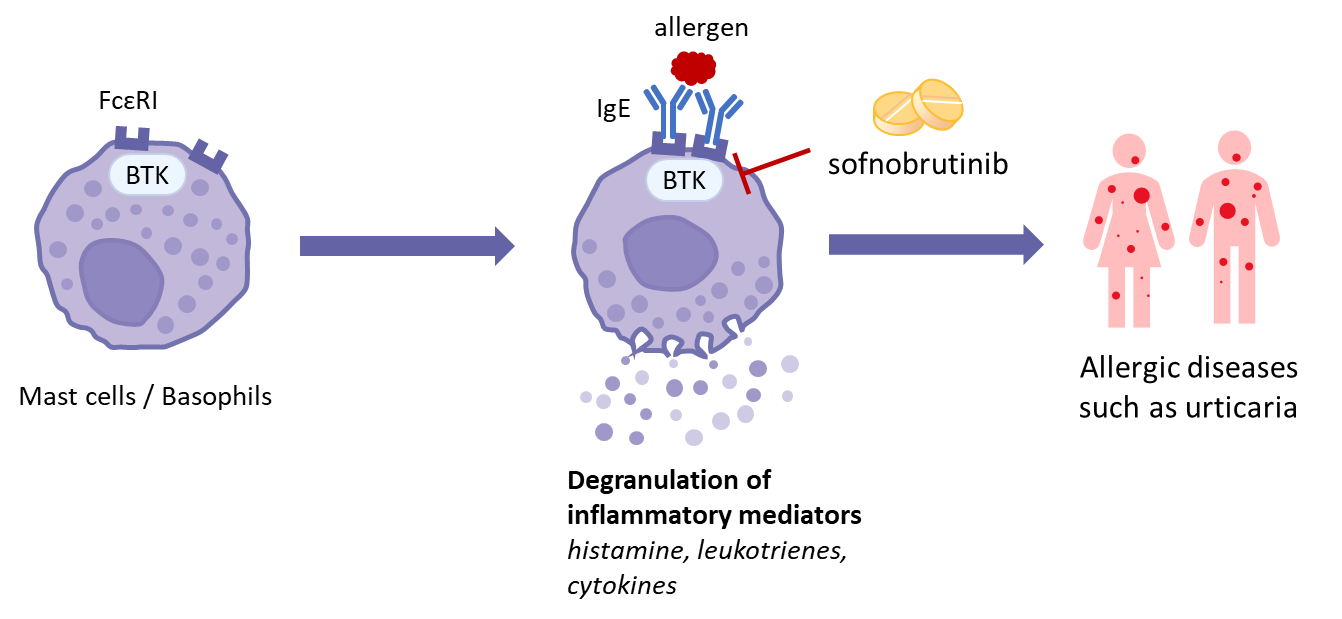
Preclinical Study
Sofnobrutinib is a non-covalent BTK inhibitor designed to bind preferentially to non-activated form of BTK protein. Sofnobrutinib is an extremely selective inhibitor with the potential to exert minimal side effects. Sonobrutinib exhibited strong inhibition of inflammatory reactions in passive cutaneous anaphylaxis (PCA) models and showed significant efficacy in collagen-induced arthritis (CIA) models.
Clinical Study
The Phase 1 clinical study of oral sofnobrutinib in heathy volunteers, consists of single ascending dose (SAD) study and multiple ascending dose (MAD) study, was conducted in the Netherlands. In the SAD study, sofnobrutinib was safe and well-tolerated at all dose levels up to 900 mg and showed favorable pharmacokinetic (PK) profile. Sofnobrutinib achieved plasma level to demonstrate pharmacodynamic (PD) effects with strong inhibition of anti-IgD-induced CD69 expression in naïve B cells and anti-IgE-induced CD63 expression in basophils at doses of 100 mg and above. In the MAD study with a new formulation, sofnobrutinib was safe and well tolerated at all dose levels and the exposure levels increased dose-dependently. Also, sofnobrutinib showed robust pharmacodynamics effect, achieved ≥90% inhibition of basophil activation at 150 mg and 300 mg twice daily. The data from the Phase 1 study support further investigation of sofnobrutinib in Phase 2 study.
References
- Design and Synthesis of Novel Amino-triazine Analogues as Selective Bruton's Tyrosine Kinase Inhibitors for Treatment of Rheumatoid Arthritis. J Med Chem. 2018 Oct 11;61(19):8917-8933.
- Design and synthesis of novel pyrimidine analogs as highly selective, non-covalent BTK inhibitors. Bioorg Med Chem Lett. 2018 Jan 15;28(2):145-151.
- 'Turn On/Off' fluorescence probe for the screening of unactivated Bruton's tyrosine kinase. Bioorg Med Chem Lett. 2015;25(10):2141-2145.
- TR-FRET binding assay targeting unactivated form of Bruton's tyrosine kinase. Bioorg Med Chem Lett. 2015;25(10):2033-2036.
