- Home
- Kinase Assay Support Portal
- Kinase Assay Support Information
- SPR Kinase Assay Support
SPR Kinase Assay Support
Surface Plasmon Resonance (SPR) technology provides data for understanding the biophysical characteristics of hits, leads and drugs. These include kinetic information such as association rate constant (ka) and dissociation rate constant (kd), as well as the binding affinity equilibrium dissociation constant (KD).
SPR (Surface Plasmon Resonance) is a technique that allows real-time observation of molecular binding events. Ligands immobilized on the surface of a biochip each have a unique resonance angle, which shifts when other molecules bind to them. By measuring changes in this resonance angle, molecular interactions such as binding and dissociation can be visualized and analyzed.
With advancements in technology, modern SPR instruments have become more sophisticated and high-throughput, enhancing their practical utility. However, in SPR measurements targeting kinases, a key challenge remains: stably immobilizing the proteins on the plate surface while maintaining their enzymatic activity.
- Reasons to Recommend Carna’s Biotinylated Proteins as SPR Measurement Reagents
- The Value of Affinity Screening Using HT-SPR (High Throughput Surface Plasmon Resonance)
- Reference Publications on SPR Assay Conditions Using Carna's Biotinylated Proteins
Reasons to Recommend Carna’s Biotinylated Proteins as SPR Measurement Reagents
Efficient and Homogenenous: Carna's biotinylated proteins, labeled with a single biotin molecule at the N-terminus, maintain the exceptionally high affinity to streptavidin (see the image on the righttop), but also create a homogenous presentation on the surface allowing assessment of interaction through first order kinetics. This makes them well-suited for constructing assay systems with minimal non-specific binding.
High Purity: All biotinylated proteins provided by Carna are offered at a purity level of over 80%, ensuring minimal concern about interference from impurities.
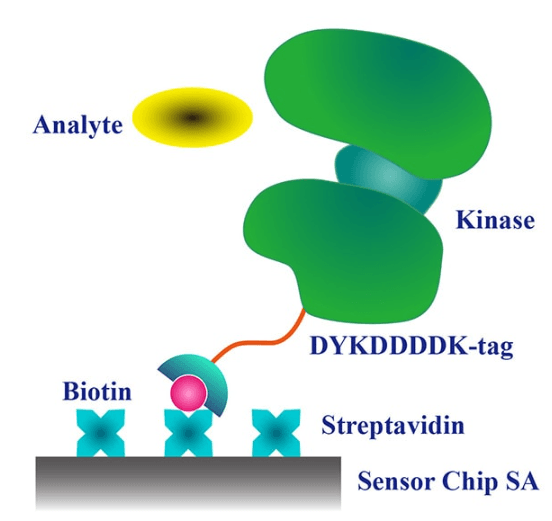
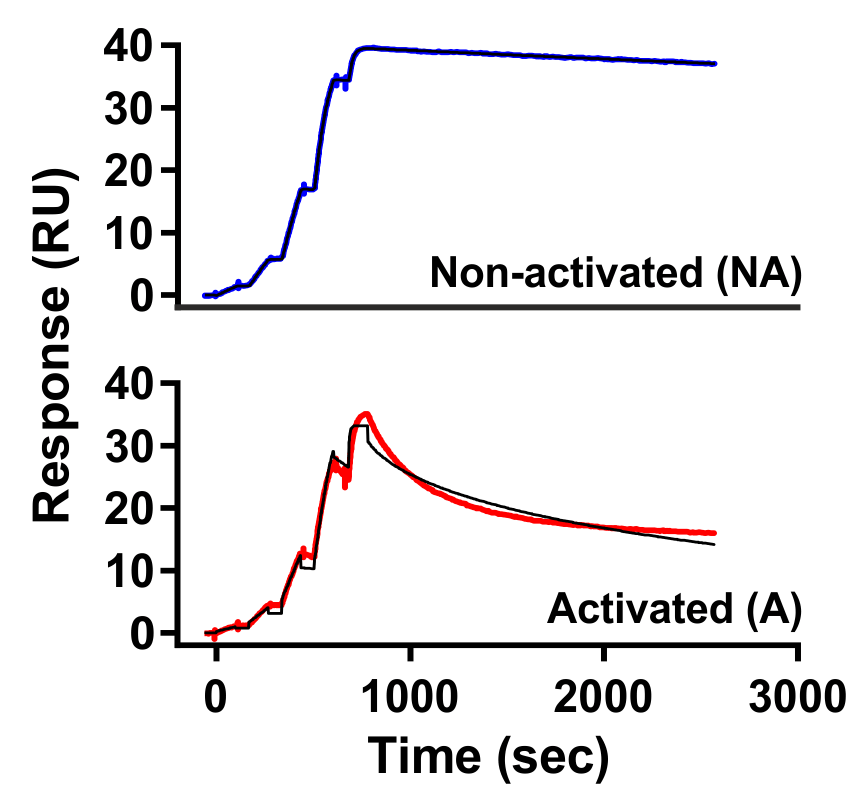
Comparative Evaluation Based on Activity States: Some of Carna’s biotinylated proteins are enhanced through ATP treatment to increase their activity. This allows for comparative evaluation with untreated, inactive products.
(Case Study on right : Fenebrutinib vs. BTK activated and non-activated)
Validated Data Available:For most of our biotinylated protein products, SPR data with existing drugs has been obtained, eliminating the need to verify reagent performance from scratch. This helps reduce both preparation time and experimental costs.
For product inquiries, questions, or consultations, please contact us from here or at info@sb.carnabio.com.
The Value of Affinity Screening Using HT-SPR (High Throughput Surface Plasmon Resonance)
In early-stage screening, incorporating orthogonal assays, such as kinetic evaluation, provides confirmation of primary enzymatic activity measurements, which leads to more confident selection of hits and leads and can help reduce development risks. Some compounds, while not directly inhibiting enzymatic activity, may still strongly bind to the target and modulate its enzymatic function. Such compounds can be harder to identify in activity-based screening alone but can be clearly detected through affinity evaluation. Additionally, obtaining binding affinity and kinetic values—in particular dissociation rates—at an early stage can contribute to a more efficacious drug in vivo.
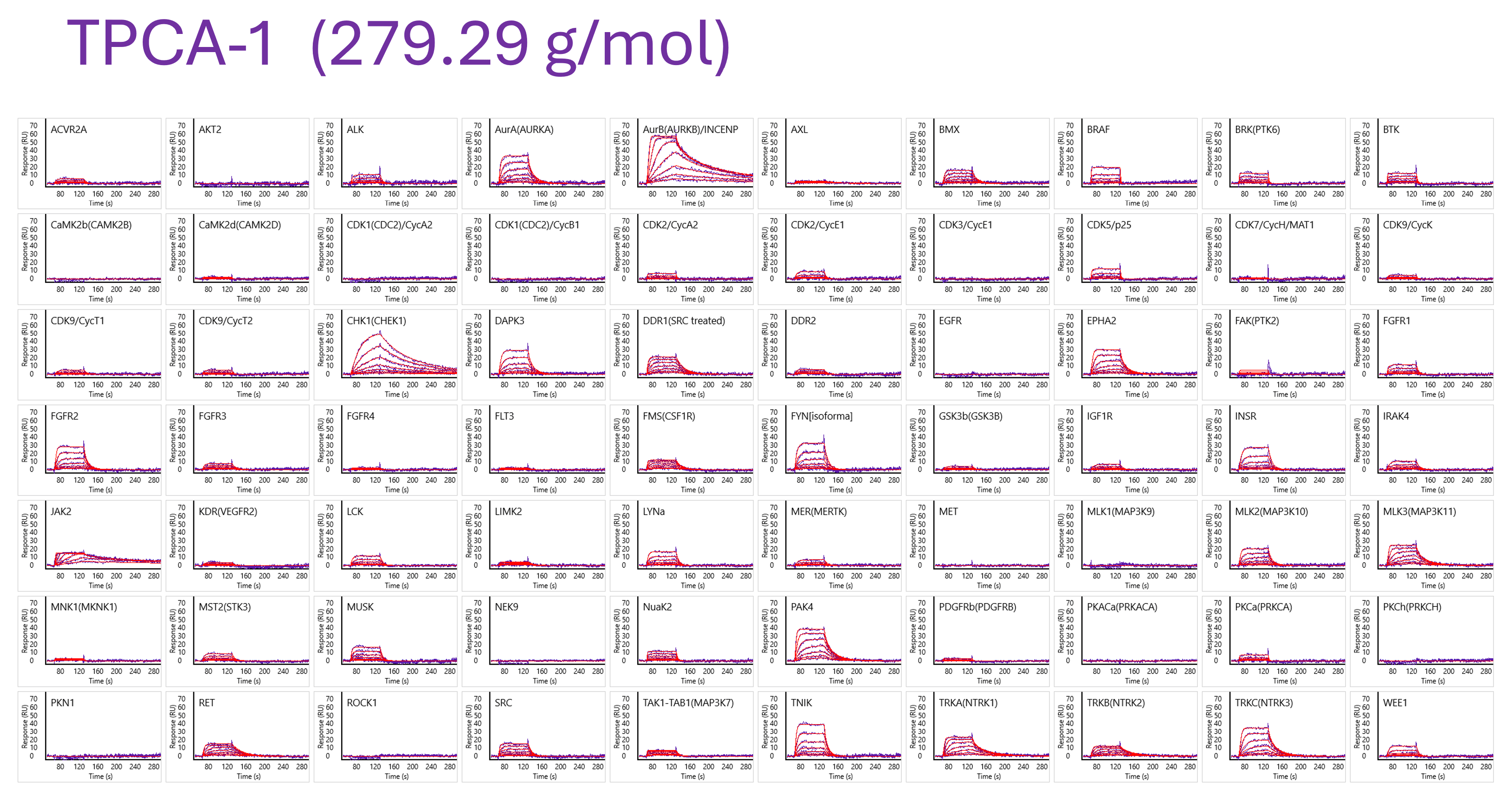
In high-throughput screening conducted using the ULTRA HT-SPR platform (Carterra), more than 80,000 unique interaction data points were obtained within just three days from combinations of 104 kinases and 210 compounds.
- Deep characterization of binding kinetics for 210 kinase inhibitors against 80+ kinases
- From Fragments to Molecules to Approved Drugs: How the Carterra Ultra HT-SPR and CarnaBio’s biotinylated kinome make kinase inhibitor discovery and optimization easy
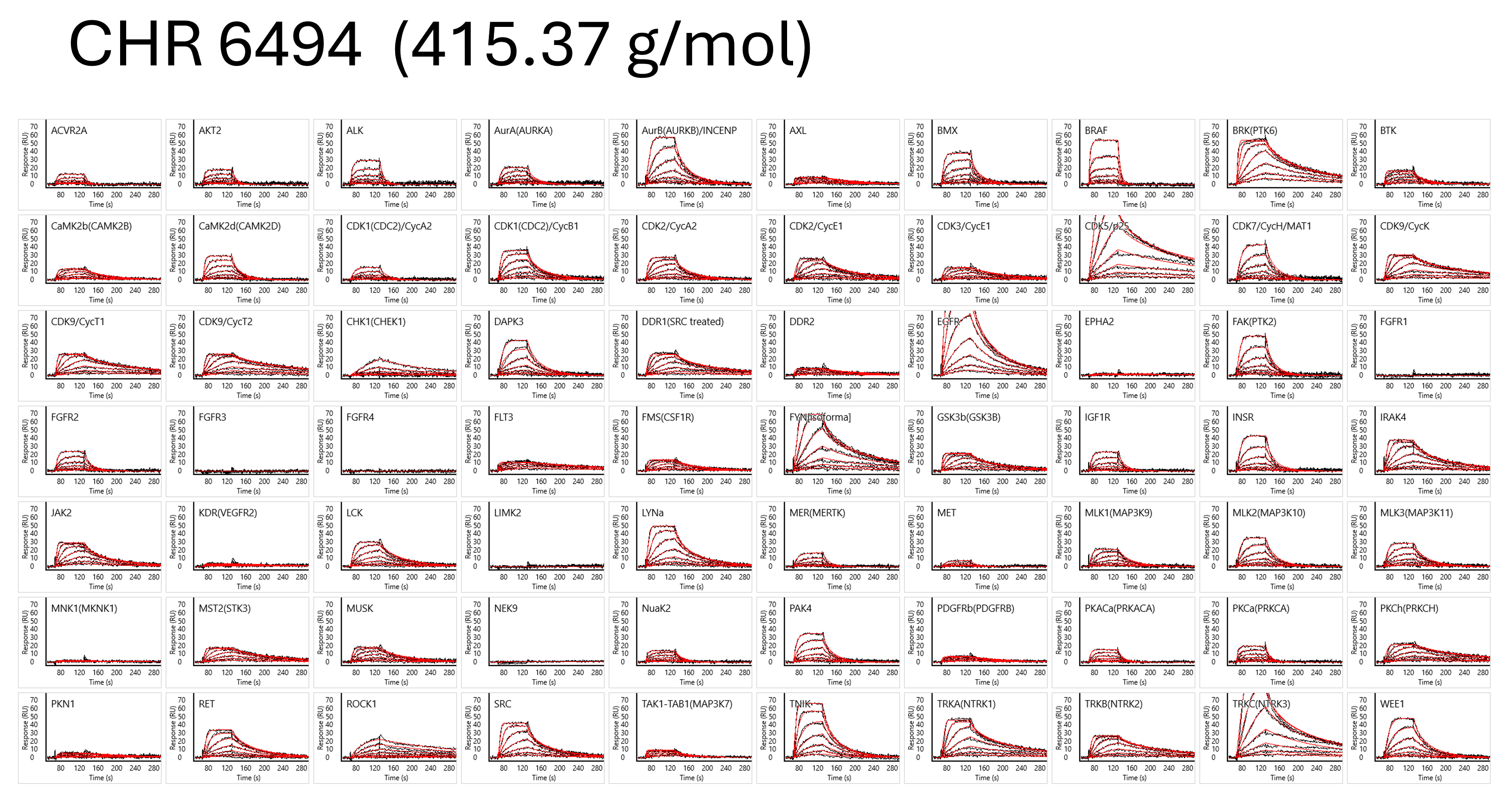
Reference Publications on SPR Assay Conditions Using Carna's Biotinylated Proteins
Journal of Molecular Biology, February 2017, 429, Issue 4, 574-586
Compound Selectivity and Target Residence Time of Kinase Inhibitors Studied with Surface Plasmon Resonance.
Willemsen-Seegers N1, Uitdehaag JCM1, Prinsen MBW1, de Vetter JRF1, de Man J1, Sawa M2, Kawase Y2, Buijsman RC1, Zaman GJR3.
Journal of Biomolecular Screening, March 2014, vol. 19 no. 3, 453-461
Quick Evaluation of Kinase Inhibitors by Surface Plasmon Resonance Using Single-Site Specifically Biotinylated Kinases
Daisuke Kitagawa, Masaki Gouda, Yasuyuki Kirii
