- Home
- Cell-Based Assay Services
- Protein-Protein Interaction Detection Assay
Protein-Protein Interaction Detection Assay
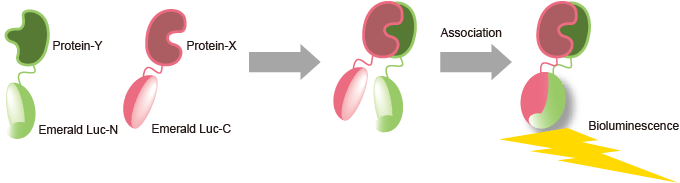
Our split luciferase complementation assay utilizing a unique luciferase derived from Pyrearinus termitilluminans (Emerald Luciferase, E-Luc) is a valuable tool for for your study of Protein-Protein Interactions (PPI). This enables detection of various types of PPIs, including GPCRs, with ease and high-sensitivity.
In addition to established cell lines on the list, we develop custom stable transfected cell lines suitable to detect specific PPIs of your interest!
Split Luciferase Technology
E-Luc is known to emit a brighter and more stable signal than conventional firefly luciferases. The N- and C-terminal domains of luciferase can be separated into two fragments, which can re-associate in cells. When those two fragments of the fused reporter proteins are brought within proximity, they spontaneously refold and generate a detectable signal (patent filed).

Pyrearinus termitilluminans and Bioluminescence
Photo Credit: Courtesy of Dr. Yoshihiro Ohmiya, The National Institute of Advanced Industrial Science and Technology (AIST)
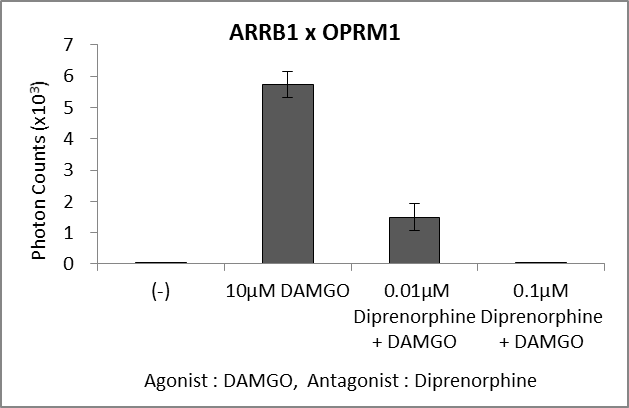
Ligand; DAMGO, 10μM/
Antagonist; Diprenorphine, 0.1μM
DAMGO stimulation of ORPM1-Eluc-C and ELucN-b-arrstin 1 co-expressing HEK293 cells in the presence or absence of diprenorphine
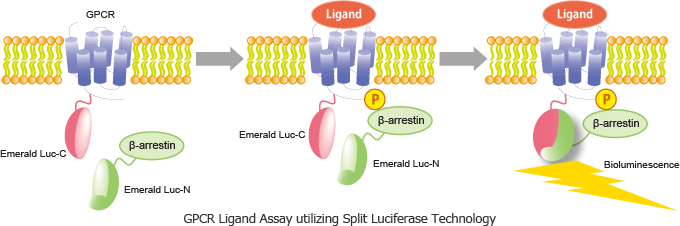
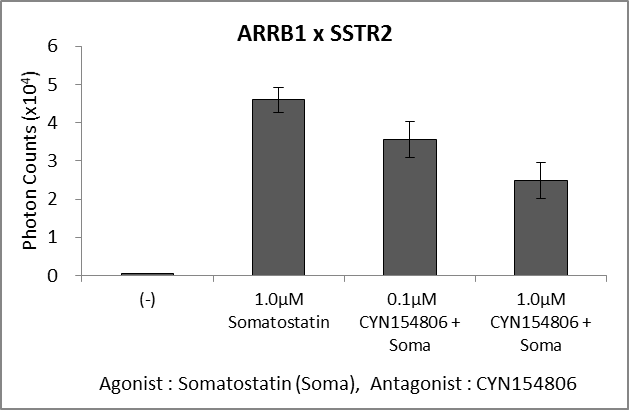
Application for GPCR
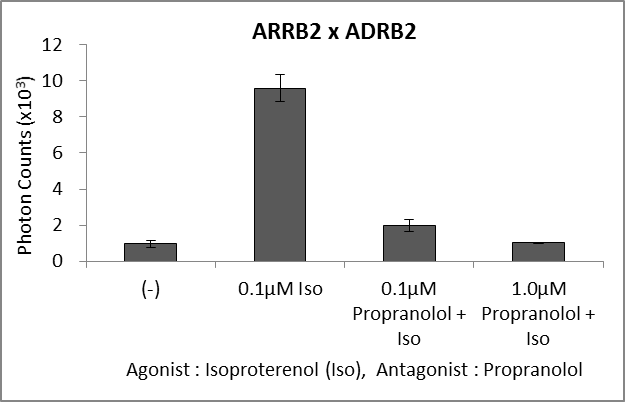
The N-terminal and C-terminal fragments of the split luciferase are fused to β-arrestin and GPCR, respectively. Binding of a ligand to GPCR triggers phosphorylation of the GPCR, thereby inducing its interaction with β-arrestin. This interaction brings the N-terminal luciferase into proximity with the C-terminal luciferase, and bioluminescence activity is measurable.
We can develop transfected cell lines not listed on the Stable Cell Line List upon request.
RAF Dimerization Custom Assay Services
The impact of your compounds on any or all of the five isoform pairs, BRAF/BRAF, BRAF/BRAF[V600E], CRAF/BRAF, CRAF/BRAF[V600E], CRAF/CRAF, can now be interrogated using our RAF dimerization assay individually customized to meet your investigation. Evaluation of RAF dimerization promoted by an inhibitor provide clues for understanding the mechanism of inhibitor-induced dimerization. Quantifying the inhibitory activity of your compounds in an assay where RAF dimerization is induced by a ligand is also possible.
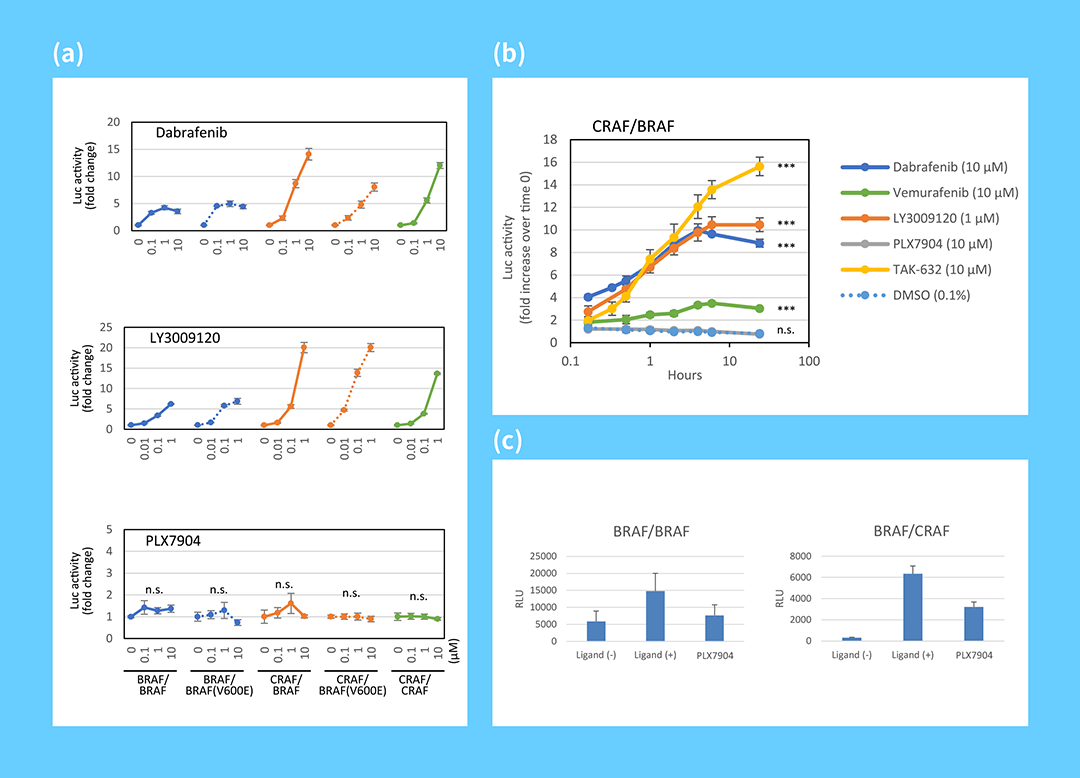
(a) Quantatative analysis of RAF dimer induction by RAF inhibitors using split luciferase probes
(b) Time course measurement of RAF dimerization promoted by RAF inhibitors
(c) Quantatative analysis of RAF dimer inhibition by PLX7904
